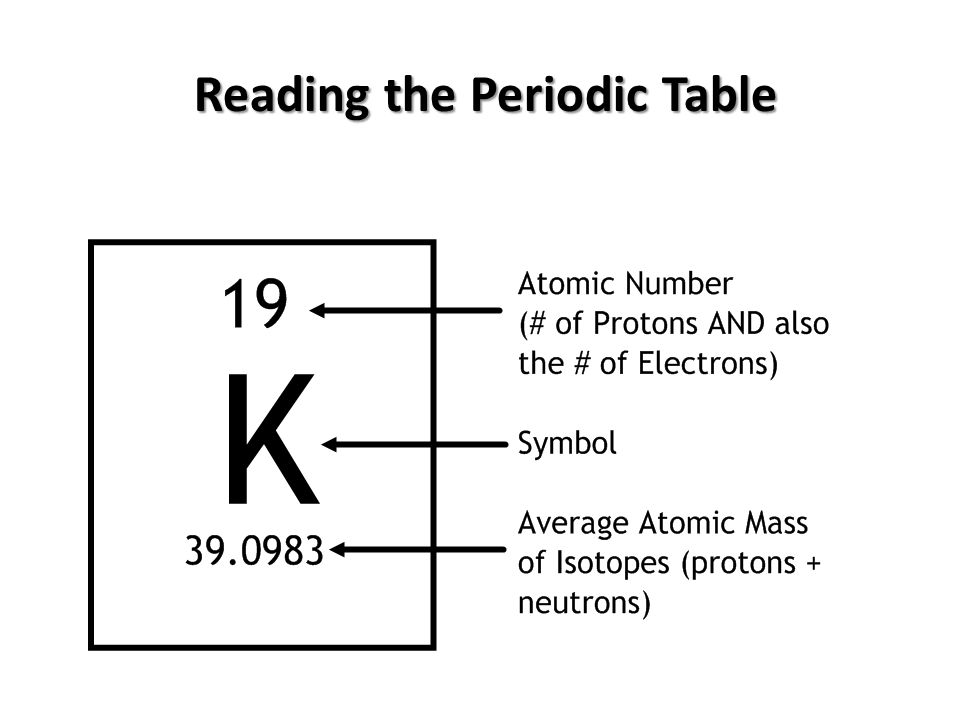
In this unit, we started by learning about biochemistry, and why it's really important to know some chemistry as a biologist. The nature of matter was discussed, as well as protons(positive), neutrons(neutral), and electrons(negative). I also learned that an element is a pure substance with only one type of atom where an atom is the basic unit of matter. The different kinds of bonds are ionic bonds, which form when an atom gains or loses an electron, and covalent bonds, which form when electrons are shared between atoms. We also learned about water, and its attraction with cohesion, attraction between the same substance, and adhesion, attraction between different substances, and capillary action, which is cohesion and adhesion.
We also learned about the properties of water, a substance necessary for life. Water is polar, so there is an unequal distribution of charge between H and O molecules, and polar substances are attracted to each other, and the strong hydrogen bonding, down to a molecular scale, has a stronger gravitational pull than the earth on an object.
 |
| The molecular structure of Sucrose, a disaccharide consisting of fructose and glucose, thus having 2 rings. |
Also, mixtures were discussed, where solutes are the things being dissolved, and solvents are what they are being dissolved in. A solution is when all compounds are evenly distributed throughout, and a suspension is a mixture of water and an undissolvable material because pieces are so small they do not settle out. Also the pH scale was described, where a number less than seven is acidic, greater than seven is basic, and equal to seven in neutral. Lastly, we learned that water is neutral, a hydrogen ion is positively charged with an ion, and a hydroxide ion is negatively charged with an electron.
The greater portion of this unit was spent explaining the four macromolecules of life, which are Carbohydrates, Lipids, Proteins, and nucleic acids. Carbohydrates are sugars(saccharides) that store energy for producers and provide energy for consumers, and they come in a ring form of carbon, hydrogen, and oxygen. Also, one thing that I really was interested in was how HFCS (high fructose corn syrup) acts as a poison to the body, and is found everyday foods. Now I am more conscious about the food I am eating, and how dangerous processed foods can be.
Then there are lipids, nonpolar molecules, that are made of long chains of hydrogen and oxygen, which are found mostly in fats and oils that store energy, make up cell membranes, and make hormones. We also talked about nucleic acids, which are made up of nucleotides that are built of a 5 carbon sugar, a phosphate group, and a nitrogen based molecule.They bond together to make 1, or 2 strands that form DNA, RNA, and ATP. Proteins like enzymes,which act as catalysts, speed up chemical reactions while lowering activation energy, and structural proteins, the building blocks of our body, are both extremely important for carrying out bodily functions. Sudden changes in pH and temperature can affect an enzyme by making it deformed in the process of denaturation. All in all, proteins, the polymers of amino acid monomers, support the body in numerous ways.
Overall, I think I can use this exposition to biology as a guide to help me understand the basics that I need to understand for labs. The labs I completed applied the basic knowledge I had gained from the lesson, while also incorporating the important details that were implored as well. I feel like I was successful at understanding the material over time, by asking questions and actively participating in class, because at first glance it was hard to comprehend the topics from just the vodcast notes. Also, the labs were both fun and interesting, which was a key factor to the active participation and its effect.
"Enzyme Active Site and Substrate Specificity - Boundless Open Textbook." Boundless. N.p., n.d. Web. 24 Sept. 2016. <https://www.boundless.com/biology/textbooks/boundless-biology-textbook/metabolism-6/enzymes-72/enzyme-active-site-and-substrate-specificity-350-11576/>.
"Fructose." Wikipedia. Wikimedia Foundation, n.d. Web. 24 Sept. 2016. <https://en.wikipedia.org/wiki/Fructose>.
"PH." Wikipedia. Wikimedia Foundation, n.d. Web. 24 Sept. 2016. <https://simple.wikipedia.org/wiki/PH>.
"Hydrolysis." Wikipedia. Wikimedia Foundation, n.d. Web. 24 Sept. 2016. <https://en.wikipedia.org/wiki/Hydrolysis>.
"Physical Science: The Structure of Matter Review Part Two." . N.p., n.d. Web. 24 Sept. 2016. <http://slideplayer.com/slide/5962551/>.